Generic Name: potassium citrate (poe TASS see um SIT rate)
Brand Names: Urocit-K
Medically reviewed by Drugs.com. Last updated on April 2, 2021.
What is potassium citrate?
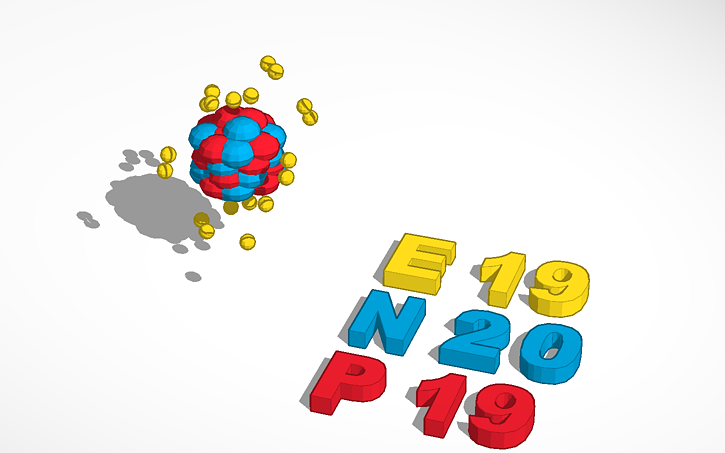
Potassium is a chemical element with atomic number 19 which means there are 19 protons and 19 electrons in the atomic structure. The chemical symbol for Potassium is K. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons. Our potassium page has over 280 facts that span 109 different quantities. Each entry has a full citation identifying its source. Areas covered include atomic structure, physical properties, atomic interaction, thermodynamics, identification, atomic size, crystal structure, history, abundances, and nomenclature.
Potassium is a mineral that is found in many foods and is needed for several functions of your body, especially the beating of your heart.
Potassium citrate is used to treat a kidney stone condition called renal tubular acidosis.
Potassium citrate may also be used for other purposes other than those listed in this medication guide.
Warnings
You should not use potassium citrate if you have kidney failure, a urinary tract infection, uncontrolled diabetes, a peptic ulcer in your stomach, Addison's disease, severe burns or other tissue injury, if you are dehydrated, if you take certain diuretics (water pills), or if you have high levels of potassium in your blood (hyperkalemia).You should not take potassium citrate tablets if you have problems with your esophagus, stomach, or intestines that make it difficult for you to swallow or digest pills.
Do not crush, chew, break, or suck on an extended-release tablet. Swallow the pill whole. Breaking or crushing the pill may cause too much of the drug to be released at one time. Sucking on a potassium tablet can irritate your mouth or throat. Avoid lying down for at least 30 minutes after you take this medication. Take potassium citrate with a meal or bedtime snack, or within 30 minutes after a meal.To be sure this medication is helping your condition, your blood may need to be tested often. Your heart rate may also be checked using an electrocardiograph or ECG (sometimes called an EKG) to measure electrical activity of the heart. This test will help your doctor determine how long to treat you with potassium. Do not miss any scheduled appointments.
Serious side effects of potassium citrate include uneven heartbeat, muscle weakness or limp feeling, severe stomach pain, and numbness or tingling in your hands, feet, or mouth.
Do not stop taking potassium citrate without first talking to your doctor. If you stop taking potassium suddenly, your condition may become worse.Before taking this medicine
You should not use this medication if you are allergic to it, or if you have certain conditions. Be sure your doctor knows if you have:high levels of potassium in your blood (hyperkalemia);
kidney failure;
a urinary tract infection;
untreated or uncontrolled diabetes;
Addison's disease (an adrenal gland disorder);
a large tissue injury such as a severe burn;
a peptic ulcer in your stomach;
if you are severely dehydrated; or
if you are taking a 'potassium-sparing' diuretic (water pill) such as amiloride (Midamor, Moduretic), spironolactone (Aldactone, Aldactazide), triamterene (Dyrenium, Dyazide, Maxzide).
You should not take potassium citrate tablets if you have problems with your esophagus, stomach, or intestines that make it difficult for you to swallow or digest pills.
Before using potassium citrate, tell your doctor if you are allergic to any drugs, or if you have:
- kidney disease;
congestive heart failure, enlarged heart, or history of heart attack;
other heart disease or high blood pressure;
diabetes;
a blockage in your stomach or intestines; or
chronic diarrhea (such as ulcerative colitis, Crohn's disease).
If you have any of these conditions, you may need a dose adjustment or special tests to safely take potassium citrate.
FDA pregnancy category C. This medication may be harmful to an unborn baby. Tell your doctor if you are pregnant or plan to become pregnant during treatment. It is not known whether potassium passes into breast milk or if it could harm a nursing baby. Do not use potassium citrate without telling your doctor if you are breast-feeding a baby.How should I take potassium citrate?
Take potassium citrate exactly as prescribed by your doctor. Do not take it in larger amounts or for longer than recommended. Follow the directions on your prescription label.
Do not crush, chew, break, or suck on an extended-release tablet. Swallow the pill whole. Breaking or crushing the pill may cause too much of the drug to be released at one time. Sucking on a potassium tablet can irritate your mouth or throat. Call your doctor if it feels like the tablet is getting stuck in your throat when you swallow it.Measure the liquid medicine with a special dose-measuring spoon or cup, not a regular table spoon. If you do not have a dose-measuring device, ask your pharmacist for one.
 Liquid potassium should be mixed with at least 4 ounces (one-half cup) of cold water or fruit juice. Drink the mixture slowly, over 5 to 10 minutes in all. To make sure you get the entire dose, add a little more water to the same glass, swirl gently and drink right away.
Liquid potassium should be mixed with at least 4 ounces (one-half cup) of cold water or fruit juice. Drink the mixture slowly, over 5 to 10 minutes in all. To make sure you get the entire dose, add a little more water to the same glass, swirl gently and drink right away.Take potassium citrate with a meal or bedtime snack, or within 30 minutes after a meal.
Your treatment may include a special diet. It is very important to follow the diet plan created for you by your doctor or nutrition counselor. You should become very familiar with the list of foods you should eat or avoid to help control your condition.To be sure this medication is helping your condition, your blood may need to be tested often. Your heart rate may also be checked using an electrocardiograph or ECG (sometimes called an EKG) to measure electrical activity of the heart. This test will help your doctor determine how long to treat you with potassium. Do not miss any scheduled appointments.
Do not stop taking potassium citrate without first talking to your doctor. If you stop taking potassium suddenly, your condition may become worse. Store potassium citrate at room temperature away from moisture and heat. Keep the medication in a closed container.What happens if I miss a dose?
Take the missed dose as soon as you remember. If it is almost time for your next dose, wait until then to take the medicine and skip the missed dose. Do not take extra medicine to make up the missed dose.
What happens if I overdose?
Seek emergency medical attention if you think you have used too much of this medicine.Overdose symptoms may include heavy feeling in your arms or legs, muscle weakness, limp feeling, slow or uneven heartbeat, chest pain, or feeling like you might pass out.
What should I avoid?
Avoid lying down for at least 30 minutes after you take potassium citrate.Avoid taking potassium supplements or using other products that contain potassium without first asking your doctor. Salt substitutes or low-salt dietary products often contain potassium. If you take certain products together you may accidentally get too much potassium. Read the label of any other medicine you are using to see if it contains potassium.
While taking this medication, avoid strenuous exercise if you are not in proper condition for it.
Potassium citrate side effects
Get emergency medical help if you have any of these signs of an allergic reaction: hives; difficulty breathing; swelling of your face, lips, tongue, or throat. Stop using potassium citrate and call your doctor at once if you have any of these serious side effects:confusion, anxiety, feeling like you might pass out;
uneven heartbeat;
extreme thirst, increased urination;
leg discomfort;
muscle weakness or limp feeling;
numbness or tingly feeling in your hands or feet, or around your mouth;
severe stomach pain, ongoing diarrhea or vomiting;
black, bloody, or tarry stools; or
coughing up blood or vomit that looks like coffee grounds.
Less serious side effects may include:
mild nausea or upset stomach;
mild or occasional diarrhea; or
appearance of a potassium citrate tablet in your stool.
This is not a complete list of side effects and others may occur. Tell your doctor about any unusual or bothersome side effect. You may report side effects to FDA at 1-800-FDA-1088.
What other drugs will affect potassium citrate?

The following drugs can interact with potassium citrate. Tell your doctor if you are using any of these:
eplerenone (Inspra);
digoxin (digitalis, Lanoxin);
candesartan (Atacand), losartan (Cozaar, Hyzaar), valsartan (Diovan), or telmisartan (Micardis);
glycopyrrolate (Robinul);
mepenzolate (Cantil);
quinidine (Quinaglute, Quinidex, Quin-Release);
atropine (Donnatal, and others), benztropine (Cogentin), dimenhydrinate (Dramamine), methscopolamine (Pamine), or scopolamine (Transderm-Scop);
a bronchodilator such as ipratroprium (Atrovent) or tiotropium (Spiriva);
bladder or urinary medications such as darifenacin (Enablex), flavoxate (Urispas), oxybutynin (Ditropan, Oxytrol), tolterodine (Detrol), or solifenacin (Vesicare);
irritable bowel medications such as dicyclomine (Bentyl), hyoscyamine (Anaspaz, Cystospaz, Levsin, and others), or propantheline (Pro-Banthine);
an ACE inhibitor such as benazepril (Lotensin), captopril (Capoten), fosinopril (Monopril), enalapril (Vasotec), lisinopril (Prinivil, Zestril), moexipril (Univasc), perindopril (Aceon), quinapril (Accupril), ramipril (Altace), or trandolapril (Mavik); or
any type of diuretic (water pill) such as bumetanide (Bumex), chlorothiazide (Diuril), chlorthalidone (Hygroton, Thalitone), ethacrynic acid (Edecrin), furosemide (Lasix), hydrochlorothiazide (HCTZ, HydroDiuril, Hyzaar, Lopressor, Vasoretic, Zestoretic), indapamide (Lozol), metolazone (Mykrox, Zarxolyn), or torsemide (Demadex).
This list is not complete and there may be other drugs that can interact with potassium citrate. Tell your doctor about all your prescription and over-the-counter medications, vitamins, minerals, herbal products, and drugs prescribed by other doctors. Do not start a new medication without telling your doctor.
Copyright 1996-2021 Cerner Multum, Inc. Version: 2.04.More about potassium citrate
Consumer resources
Professional resources
Related treatment guides
Further information
Remember, keep this and all other medicines out of the reach of children, never share your medicines with others, and use potassium citrate only for the indication prescribed.

Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
Mass Of Potassium
The Element Potassium
Potassium is a chemical element in the periodic table that has the symbol K (Latin, Kalium) and atomic number 19. This is a soft, silvery-white metallic alkali metal that occurs naturally bound to other elements in seawater and many minerals. It oxidizes rapidly in air, is very reactive, especially in water, and resembles sodium chemically.
Notable characteristics
Potassium is the second lightest metal. It is a soft solid that easily is cut with a knife and is silvery in color on fresh surfaces. It oxidizes in air rapidly and must be stored in mineral oil for preservation.
Similar to other alkali metals, potassium reacts violently with water producing hydrogen. When in water it may catch fire spontaneously. Its salts emit a violet color when exposed to a flame.
Applications
- Potassium oxide, best known as potash, is primarily used in fertilizer.
- Potassium nitrate is used in gunpowder.
- Potassium carbonate is used in glass manufacture.
- NaK an alloy of sodium and potassium is used as a heat-transfer medium.
- This element is an essential component needed in plant growth and is found in most soil types.
- In animal cells potassium ions are vital to keeping cells alive (see Na-K pump)
- Potassium chloride is used as a substitute for table salt and is also used to stop the heart, e.g. in cardiac surgery and in executions by lethal injection.
Many potassium salts are very important, and include, potassium; bromide, carbonate, chlorate, chloride, chromate, cyanide, dichromate, hydroxide, iodide, nitrate, sulfate.
History
Potassium (English, potash L. kalium) was discovered in 1807 by Sir Humphry Davy who derived it from caustic potash (KOH. This alkali metal was the first metal that was isolated by electrolysis.
Occurrence
This element makes up about 2.4% of the weight of the Earth's crust and is the seventh most abundant element in it. Due to its insolubility, it is very difficult to obtain potassium from its minerals.
However, other minerals, such as carnallite, langbeinite, polyhalite, and sylvite are found in ancient lake and sea beds. These minerals form extensive deposits in these environments, making extracting potassium and its salts more economical. The principle source of potassium, potash is mined in California, Germany, New Mexico, Utah, and in other places around the world. At 3000 ft below the surface of Saskatchewan are large deposits of potash which may become important sources of this element and its salts in the future.The oceans are another source of potassium but the quantity present in a given volume of seawater is relatively low compared to sodium.

Potassium can be isolated through electrolysis of its hydroxide in a process that has changed little since Davy. Thermal methods also are employed in potassium production. Potassium is almost never found unbound in nature. However, in living organisms K+ ions are important in the physiology of excitable cells.
Isotopes
There are seventeen isotopes of potassium known to exist. The non-synthetic form of potassium are composed of three isotopes: K-39 (93.3%), K-40 (0.01%) and K-41 (6.7%). Naturally occurring K-40 decays to stable Ar-40 (11.2%) by electron capture and by positron emission, and decays to stable Ca-40 (88.8%) by negatron emission; K-40 has a half-life of 1.250 × 109 years.
Potassium Atom Size
Potassium Atomic Mass
The decay of K-40 to Ar-40 is commonly used as a method for dating rocks. The conventional K-Ar dating method depends on the assumption that the rocks contained no argon at the time of formation and that all the subsequent radiogenic argon (i.e., Ar-40) was quantitatively retained, i.e., closed system. Minerals are dated by measurement of the concentration of potassium, and the amount of radiogenic Ar-40 that has accumulated. The minerals that are best suited for dating include biotite, muscovite, and plutonic/high grade metamorphic hornblende, and volcanic feldspar; whole rock samples from volcanic flows and shallow instrusives can also be dated if they are unaltered.
